The Medical Devices Regulation (MDR) has been applicable since 26 May 2021 and it provided for a transition period until 26 May 2024. The transition of medical devices to the MDR has been slower than anticipated and healthcare systems in the EU are facing a risk of shortages. On 20 March 2023 a new EU Regulation has been adopted, namely Regulation EU 2023/607 amending the MDR and IVDR, in particular as to the timelines for the transition of certain medical devices to the MDR/IVDR, thus providing additional time to manufacturers and notified bodies to complete the transition of their devices, ensuring continued access to medical devices for patients in need. The Regulation extended the MDR transition timelines as well as the validity of the certificates issued previously on the basis of the Medical Device Directives (MDD) and was adopted in order to address the risk of shortages of medical devices in the EU due to the slower-than-anticipated transition from the MDDs to the MDR/IVDR, given the COVID-19 impact.
Hence, by operation of the new Regulation, medical devices may continue to be placed on the market in adherence to the MDDs, while simultaneously they continue their transition to the MDR/IVDR. Having said this, it must be noted that the extended transition timelines are applicable only to medical devices that are in fact transitioning to MDR/IVDR and meet other specific conditions, as these are set out in the Regulation.
Specifically, depending on the various categories of medical devices, the transition timelines are outlined below, and are applicable subject to the manufacturer submitting an MDR application by 26 May 2024, having entered into an agreement with a notified body by 26 September 2024 and meeting any other conditions as these are set out in the Regulation.
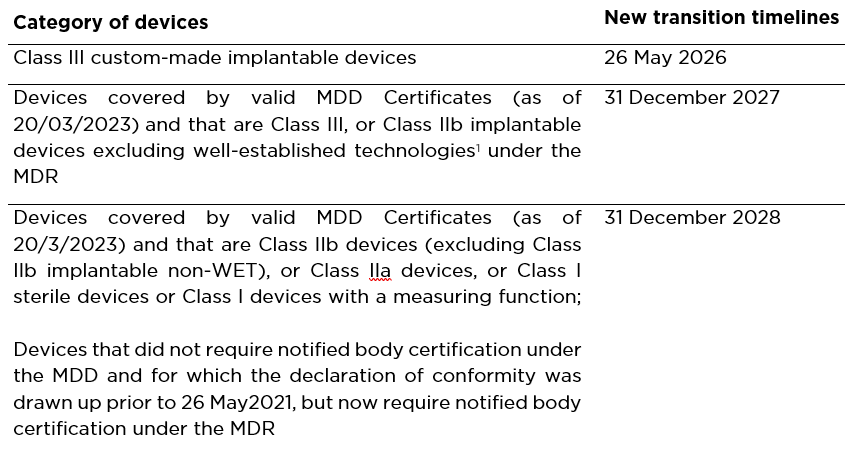
Medical devices covered by an MDD certificate that was valid as of 26 May 2021, but expired prior to the publication of the new Regulation, benefit from the extended transition timelines (as shown in the table above) only if the manufacturer had applied for MDR and signed a formal written agreement with a notified body prior to the expiry of those MDD certificates or a derogation/ exemption has been granted by a competent Authority under either Article 59(1) or Article 97(1) of the MDR. Also, Article 120(4) of the MDR has been abolished to the effect that medical devices already placed on the market under the MDDs may continue to be further available for an indefinite period.
As a general comment, it is argued that the revised timeline provided for by the new Regulation will provide more flexibility to the industry for the ongoing certification of medical devices and reduce short-term risks of shortages. This will ensure access for patients most in need without jeopardising their safety, but it is important to recall that only devices that are safe and for which manufacturers have already taken steps to transition to the MDR can benefit from this additional time. Thus, albeit additional time is now available, the Authorities strongly recommend that companies that have already filed or are about to file their MDR applications not stall and strongly urge those who are yet to make their MDR applications to submit them as soon as possible, given that only those devices transitioning to the MDR benefit from the longer transition timelines and extended validity of their MDD certificates and in case of last-minute applications there is a risk of not meeting the cut-off deadlines.
The new Regulation may be found here: Regulation (EU) 2023/607.
Also, the Directorate-General for Health and Food Safety has issued a ”Q&A on practical aspects related to the implementation of Regulation (EU) 2023/607 - Extension of the MDR transitional period and removal of the “sell off” periods” which may be found here.
_________
[1] Well-established technologies: sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors and which are all Class III or implantable devices.

